Tacrolimus Biotin Conjugate Solution
CAT:
952-C2010006
Size:
5x 1 mg
For Laboratory Research Only. Not for Clinical or Personal Use.
Price:
Ask
- Availability: 24/48H Stock Items & 2 to 6 Weeks non Stock Items.
- Dry Ice Shipment: No
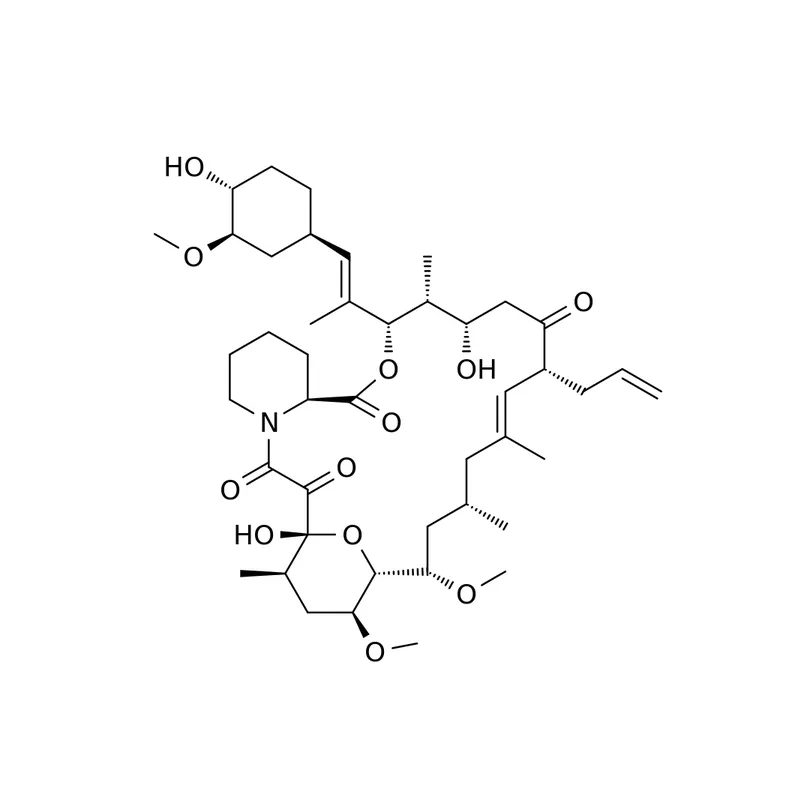







Tacrolimus Biotin Conjugate Solution
Description:
Tacrolimus Biotin Conjugate Solution_x000D_ _x000D__x000D_ _x000D_
_x000D_ _x000D_ _x000D_ Complete Tacrolimus Product Line_x000D_ _x000D_ Download Brochure_x000D_ _x000D__x000D_ _x000D_Catalog number: _x000D_C2010006 _x000D__x000D_ _x000D_Lot number: _x000D_Batch Dependent _x000D__x000D_ _x000D_Expiration date: _x000D_Batch Dependent _x000D__x000D_ _x000D_Purity: _x000D_> 95% by HPLC _x000D__x000D_ _x000D_Concentration: _x000D_10 mg/mL (in DMSO) _x000D__x000D_ _x000D_Package Size: _x000D_1 mg and 5 mg _x000D__x000D_ _x000D_Appearance: _x000D_Yellow Solution _x000D__x000D_ _x000D_Storage: _x000D_-20°. Avoid repeated freeze-thaw. _x000D__x000D_ _x000D_Other names: _x000D_Biotin-Tacrolimus, Biotin conjugated to Tacrolimus, FK506-Biotin, Biotin-FK506, Biotinylated Tacrolimus, Biotinylated FK506. _x000D__x000D_ _x000D_ _x000D_Keywords: _x000D_Biotin conjugate, Tacrolimus 32, Tacrolimus, FK-506, Fujimycin, Prograf, Advagraf, Protopic. _x000D_ _x000D_
_x000D_
References_x000D_
_x000D_
_x000D_
References_x000D_
- _x000D_
- Brunet, M., Plana, J. C., and Rimola, A. (2001). Tacrolimus. Madrid: Drug Farma. _x000D_
- Goto, T., and Nakagawa, H. (2004). Development of Tacrolimus Ointment. Tacrolimus Ointment, 81€“98. _x000D_
- Raptis, D., and Pramateftakis, M.-G. (2013). Tacrolimus: Effectiveness, safety and drug interactions. New York: Nova Biomedical. _x000D_
- Plosker, G. L., and Foster, R. H. (2001). Tacrolimus a further update of its pharmacology and therapeutic use in the management of organ transplantation. Auckland: ADIS International. _x000D_
- Kaplan, B., Burckart, G. J., and Lakkis, F. G. (2012). Immunotherapy in transplantation: principles and practice. Chichester, West Sussex, UK: Wiley Blackwell. _x000D_
- Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, Shimojo N, Tanaka A, Nakahara T, Nagao M, Hide M, Fujita Y, Fujisawa T, Futamura M, Masuda K, Murota H, Yamamoto-Hanada K; Committee for Clinical Practice Guidelines for the Management of Atopic Dermatitis 2018, Japanasese Society of Allergology, Japanese Dermatology Association. Japanese guidelines for atopic dermatitis 2020. Allergol Int. 2020 Apr 4. pii: S1323-8930(20)30018-6. _x000D_
- Lv X, Qi J, Zhou M, Shi B, Cai C, Tang Y, Pan T, Han Y. Comparative efficacy of 20 graft-versus-host disease prophylaxis therapies for patients after hematopoietic stem-cell transplantation: A multiple-treatments network meta-analysis. Crit Rev Oncol Hematol. 2020 Mar 20;150:102944. _x000D_
- Oberbauer R, Bestard O, Furian L, Maggiore U, Pascual J, Rostaing L, Budde K. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant Rev (Orlando). 2020 Apr;34(2):100531. _x000D_
Short Description:
Catalog #: C2010006 (1 mg or 5 mg)_x000D_ Tacrolimus Biotin Conjugate Solution is a concentrated solution of Tacrolimus (FK506) conjugated to Biotin through a PEG linker. Dilutions of the provided conjugate can be made in DMSO. The provided conjugate can be used in the research of Tacrolimus detection methods that include ELISA, Western Blots and other research applications. Custom bulk orders of this product are available upon request._x000D_ _x000D_ Live Enquiry about this product via Text/SMS: 1-858-900-3210 (8 am - 8 pm PST)Weight:
32Length:
8Width:
6.75Height :
5.5