Alpha Synuclein pSer129 Monomers
CAT:
400-SPR-520B
Size:
100 µg
Price:
Ask
- Availability: 24/48H Stock Items & 2 to 6 Weeks non Stock Items.
- Dry Ice Shipment: Yes



















Alpha Synuclein pSer129 Monomers
Background:
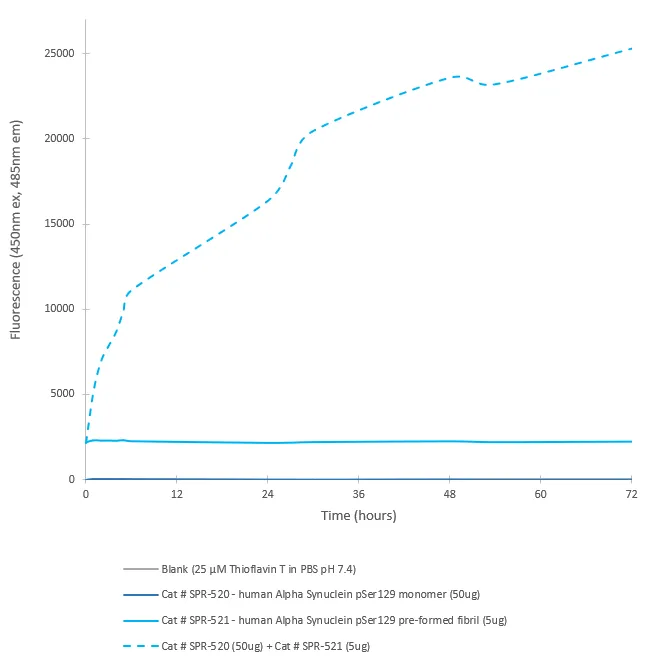
Serine 129 is the C-terminal serine characteristic to mammalian alpha synuclein, with this serine being determined to be a major phosphorylation site (1). Lewy Bodies in Parkinson’s Disease (PD) and other related synucleinopathies are comprised of alpha synuclein phosphorylated at serine 129 and this phosphorylation may contribute to an increased propensity to aggregate (2). Due to phosphorylation at serine 129 being one of the most abundant PTMs, several studies reported on the PTM as a potential biomarker (3). Our Alpha Synuclein Ser129 Monomers are generated in-house and phosphorylation confirmed with our anti-ASYN pS129 monoclonal antibody (Catalog# SMC-600).Description:
Human Recombinant Alpha Synuclein Monomers phosphorylated at position 129Product Name Alternative:
Alpha synuclein monomer, Alpha-synuclein monomer, Alpha synuclein protein monomer, Alpha synuclein monomer, Alpha-synuclein protein, Non-A beta component of AD amyloid protein, Non-A4 component of amyloid precursor protein, NACP protein, SNCA protein, NACP protein, PARK1 protein, Alpha synuclein monomers, SYN protein, Parkinson's disease familial 1 ProteinUNSPSC:
12352202Swiss Prot:
P37840Host:
E. coliOrigin Species:
HumanTarget:
Alpha Synuclein pSer129Conjugation:
No TagSequence:
MDVFMKGLSK AKEGVVAAAE KTKQGVAEAA GKTKEGVLYV GSKTKEGVVH GVATVAEKTK EQVTNVGGAV VTGVTAVAQK TVEGAGSIAA ATGFVKKDQL GKNEEGAPQE GILEDMPVDP DNEAYEMPSE EGYQDYEPEAApplications:
WB, SDS-PAGE, In vivo assay, In vitro assayPurification Method:
Ion-exchange PurifiedConcentration:
2 mg/ml or 5 mg/mlPurity:
>95%Weight:
0.01Length:
140 aaBuffer:
PBS pH 7.4Molecular Weight:
14.46 kDaPrecautions:
Not for use in humans. Not for use in diagnostics or therapeutics. For research use only.Additionnal Information:
For corresponding PFFs, see Catalog# SPR-521. The unphosphorylated construct is Catalog# SPR-321.References & Citations:
1. Okochi et al. 2000. Constitutive phosphorylation of the Parkinson’s Disease Associated a-Synuclein. The Journal of Biological Chemistry. DOI: 10.1074/jbc.275.1.390 2. Fujiwara et al. 2002. α-Synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biology. DOI: 10.1038/ncb748 3. Magalhaes and Lashuel. 2002. Opportunities and challenges of alpha-synuclein as a potential biomarker for Parkinson’s disease and other synucleinopathies. Npj Parkinsons Disease. DOI: 10.1038/s41531-022-00357-0
MSDS Document
View Document