Recombinant Human NLRP1 protein (NLRP1)
CAT:
399-CSB-EP871630HU-02
Size:
100 µg
Price:
Ask
- Availability: 24/48H Stock Items & 2 to 6 Weeks non Stock Items.
- Dry Ice Shipment: No



Recombinant Human NLRP1 protein (NLRP1)
- CAS Number: 9000-83-3
- Gene Name: NLRP1
- UniProt: Q96AM0
- Expression Region: 1-146aa
- Organism: Homo sapiens
- Target Sequence: MPLDPYHSVTWGHAQGSHHFVFGELRVRPILESLRDEPDPDPRPSREGPAGRVGALARGGPEPCDAASPPGGASCAPELARPREDKSAQQAKLEGGTRLCCRCPEESRLVPGGAVSPGDHVLEVSGTRGTCGCRPRRHAGPELAHS
- Tag: N-terminal GST-tagged
- Source: E.coli
- Field of Research: Cell Biology
- Assay Type: In Stock Protein
- Relevance: Acts as the sensor component of the NLRP1 inflammasome, which mediates inflammasome activation in response to various pathogen-associated signals, leading to subsequent pyroptosis . Inflammasomes are supramolecular complexes that assemble in the cytosol in response to pathogens and other damage-associated signals and play critical roles in innate immunity and inflammation . Acts as a recognition receptor (PRR): recognizes specific pathogens and other damage-associated signals, such as cleavage by human rhinoviruses 14 and 16 (HRV-14 and HRV-16), double-stranded RNA or Val-boroPro inhibitor, and mediates the formation of the inflammasome polymeric complex composed of NLRP1, CASP1 and PYCARD/ASC . In response to pathogen-associated signals, the N-terminal part of NLRP1 is degraded by the proteasome, releasing the cleaved C-terminal part of the protein (NACHT, LRR and PYD domains-containing protein 1, C-terminus), which polymerizes and associates with PYCARD/ASC to initiate the formation of the inflammasome complex: the NLRP1 inflammasome recruits pro-caspase-1 (proCASP1) and promotes caspase-1 (CASP1) activation, which subsequently cleaves and activates inflammatory cytokines IL1B and IL18 and gasdermin-D (GSDMD), leading to pyroptosis . Activation of NLRP1 inflammasome is also required for HMGB1 secretion; the active cytokines and HMGB1 stimulate inflammatory responses . Binds ATP and shows ATPase activity . Plays an important role in antiviral immunity and inflammation in the human airway epithelium . Specifically recognizes a number of pathogen-associated signals: upon infection by human rhinoviruses 14 and 16 (HRV-14 and HRV-16), NLRP1 is cleaved and activated which triggers NLRP1-dependent inflammasome activation and IL18 secretion . Positive-strand RNA viruses such as. Semliki forest virus and long dsRNA activate the NLRP1 inflammasome, triggering IL1B release in a NLRP1-dependent fashion . Acts as a direct sensor for long dsRNA and thus RNA virus infection . May also be activated by muramyl dipeptide (MDP), a fragment of bacterial peptidoglycan, in a NOD2-dependent manner .; [NACHT, LRR and PYD domains-containing protein 1]: Constitutes the precusor of the NLRP1 inflammasome, which mediates autoproteolytic processing within the FIIND domain to generate the N-terminal and C-terminal parts, which are associated non-covalently in absence of pathogens and other damage-associated signals.; [NACHT, LRR and PYD domains-containing protein 1, N-terminus]: Regulatory part that prevents formation of the NLRP1 inflammasome: in absence of pathogens and other damage-associated signals, interacts with the C-terminal part of NLRP1 (NACHT, LRR and PYD domains-containing protein 1, C-terminus), preventing activation of the NLRP1 inflammasome . In response to pathogen-associated signals, this part is ubiquitinated and degraded by the proteasome, releasing the cleaved C-terminal part of the protein, which polymerizes and forms the NLRP1 inflammasome .; [NACHT, LRR and PYD domains-containing protein 1, C-terminus]: Constitutes the active part of the NLRP1 inflammasome . In absence of pathogens and other damage-associated signals, interacts with the N-terminal part of NLRP1 (NACHT, LRR and PYD domains-containing protein 1, N-terminus), preventing activation of the NLRP1 inflammasome . In response to pathogen-associated signals, the N-terminal part of NLRP1 is degraded by the proteasome, releasing this form, which polymerizes and associates with PYCARD/ASC to form of the NLRP1 inflammasome complex: the NLRP1 inflammasome complex then directly recruits pro-caspase-1 (proCASP1) and promotes caspase-1 (CASP1) activation, leading to gasdermin-D (GSDMD) cleavage and subsequent pyroptosis .; [Isoform 2]: It is unclear whether is involved in inflammasome formation. It is not cleaved within the FIIND domain, does not assemble into specks, nor promote IL1B release . However, in an vitro cell-free system, it has been shown to be activated by MDP .
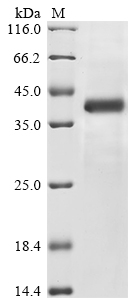
- Purity: Greater than 85% as determined by SDS-PAGE.
- Activity: Not Test
- Length: Full Length
- Form: Liquid or Lyophilized powder
- Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0.
- Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
- Molecular Weight: 42.9 kDa
- References & Citations: "Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity." Finger J.N., Lich J.D., Dare L.C., Cook M.N., Brown K.K., Duraiswami C., Bertin J.J., Bertin J., Gough P.J. J. Biol. Chem. 287:25030-25037 (2012)
- Storage Conditions: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20℃/-80℃. The shelf life of lyophilized form is 12 months at -20℃/-80℃.
