MCC-950
- Availability: 24/48H Stock Items & 2 to 6 Weeks non Stock Items.
- Dry Ice Shipment: No







MCC-950
Background:
NLRP3 (MCC950) was originally found to act as a cytokine release inhibitory drug (CRID), arresting activated monocytes and preventing activation of caspase-1(1). Discovered to be a novel inhibitor of the NLRP3 and AIM2 inflammasomes(2). Blocks canonical and noncanonical NLRP3 activation at nanomolar concentrations3. Inhibits interleukin 1β (IL-1β) secretion in vivo and attenuates the severity of experimental autoimmune encephalomyelitis (an MS disease model)(3). Disrupts the interaction between AIM2 and ASC in a reconstituted cell-free inflammasome(4). A valuable new tool for exploring the pathophysiology of NLRP3. It may contribute to additional alpha synuclein aggregation and cell loss in PD (5).Description:
NLRP3 inflammasome activation inhibitorProduct Name Alternative:
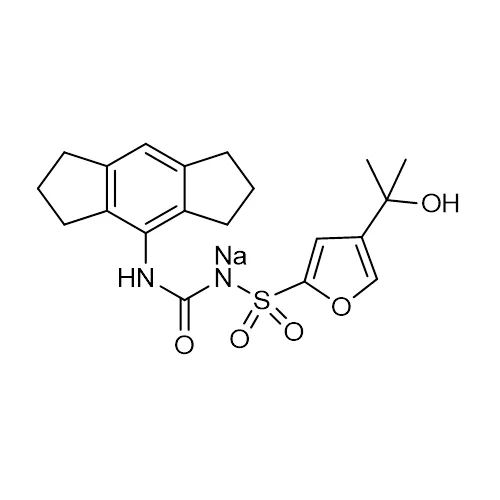
N-[[ (1,2,3,5,6,7-Hexahydro-s-indacen-4-yl) amino]carbonyl]-4- (1-hydroxy-1-methylethyl) -2-furansulfonamide sodium salt; CRID3; CP-456773 sodium saltUNSPSC:
41116105Type:
InhibitorSource:
SyntheticField of Research:
Cell Signaling | Growth Factors | TNF | Neuroscience | Neurodegeneration | Parkinson's Disease | Synuclein| CancerPurity:
>98% (HPLC) ; NMR (Conforms)Weight:
0.005Format:
White powderSolubility:
May be dissolved in water (30 mg/ml): or DMSO (40 mg/ml)Molecular Formula:
C20H23N2O5S ∙ NaMolecular Weight:
426.5Precautions:
Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only.References & Citations:
1. Laliberte R.E. et al. (2003) J. Biol. Chem. 278(19):16567-78. 2. Coll R.C., et al. (2011) PLoS One. 6(12):e29539. 3. Coll R.C., et al. (2015) Nat. Med. 21(3):248-255. 4. Kaneko N., et al. (2015) J. Immunol. Methods. 426:76-81. 5. Nguyen L., et al. (2022) J Parkinsons Dis. 12(7): 2117-2133.CAS Number:
256373-96-3
