Loading...
Cell line engineering is at the heart of modern biotechnology. Whether you're talking about life-saving monoclonal antibodies or industrial enzymes, the ability to program living cells to produce specific proteins is one of the most transformative tools in the life sciences.
One of the clearest and most widely adopted applications is engineering Chinese Hamster Ovary (CHO) cells to produce therapeutic antibodies. These modified cells are used worldwide to manufacture drugs for cancer, autoimmune diseases, and chronic inflammatory conditions.
Gentaur
Scientific Publications
CHO cells have been the workhorse of the biopharmaceutical industry for decades. Originally derived in the 1950s, they've been adapted to grow in suspension cultures and serum-free media — two crucial traits for scalability and safety. More importantly, CHO cells can perform human-compatible glycosylation, which is essential for the biological activity, stability, and immunogenicity of therapeutic proteins.
Another advantage is their regulatory history. CHO cells are well-characterized and accepted by health authorities, which streamlines the drug approval process when these cells are used in manufacturing.
At the core of the engineering process is the introduction of recombinant DNA that encodes the antibody's heavy and light chains. These genes are inserted into the cells' genome, turning them into tiny, self-replicating bioreactors.
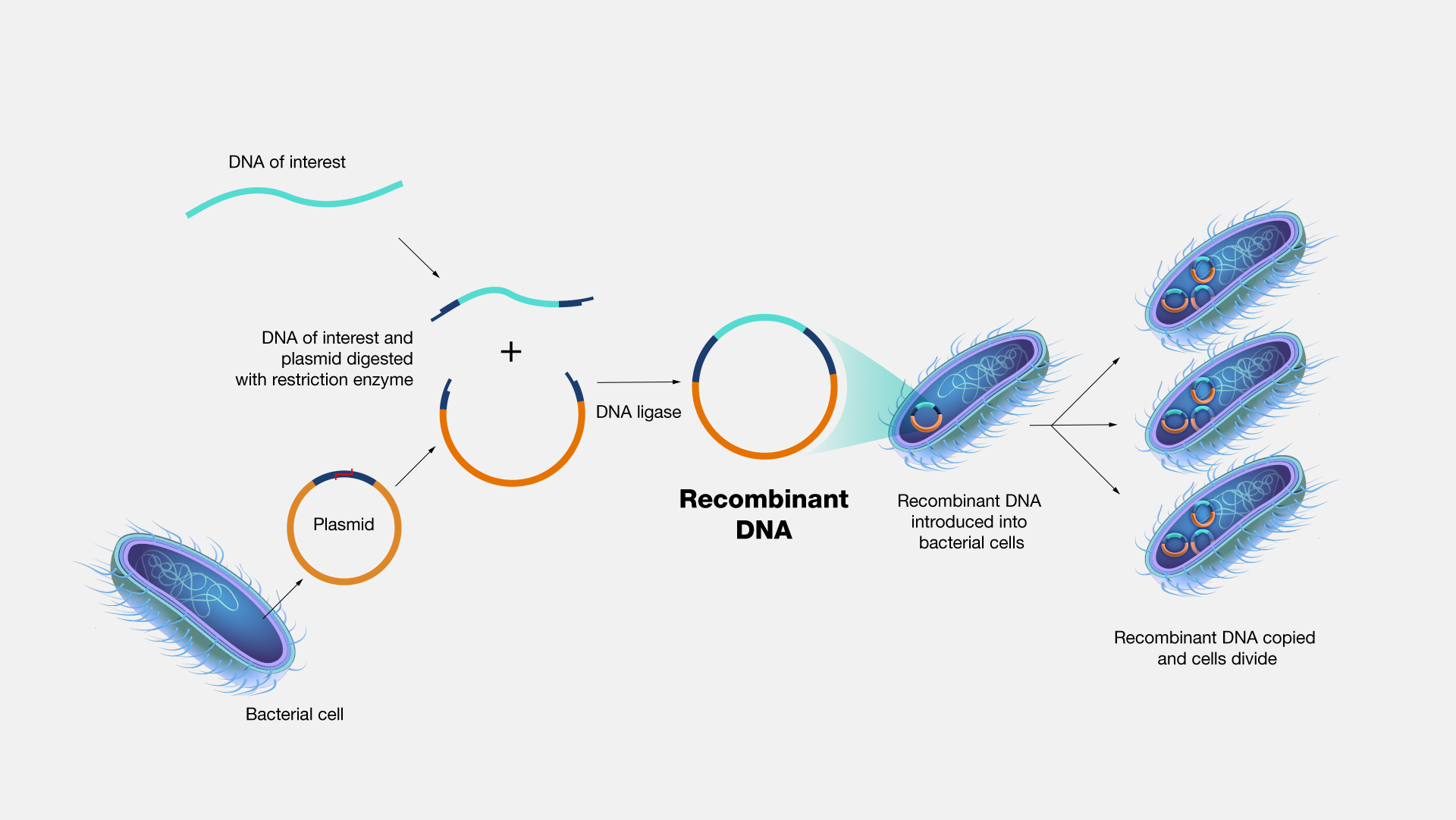
Once integrated, the cells begin producing the antibody continuously. However, not all cells perform equally. Some express high levels of the protein, others less so. Through rounds of selection and screening, researchers isolate the most productive and stable cell clones.
These clones are then expanded and validated. Stability testing ensures that they maintain their productivity across many generations a requirement for regulatory approval and consistent commercial manufacturing.
Producing large quantities of antibodies is only half the equation. The quality of the protein including its folding, glycosylation, and functional activity must meet strict pharmaceutical standards.
That's why engineered CHO cell lines undergo extensive characterization. Analytical methods such as ELISA, SDS-PAGE, HPLC, and mass spectrometry are used to confirm the identity, purity, and bioactivity of the product. Any deviations could affect its effectiveness or applicability in research and industrial settings.
Although monoclonal antibodies are the flagship product, CHO cell engineering is also used to produce hormones, cytokines, fusion proteins, and even viral vectors for gene therapy. The same fundamental principles apply: modify the cell, select for performance, and optimize production.
This versatility makes cell line engineering a foundational technology in biotechnology, supporting both basic research and advanced applications.
Cell line engineering is not just genetic manipulation — it’s the systematic design of a living production system. CHO cells have become the preferred platform because they combine reliability, scalability, and human-compatible protein processing.
By engineering these cells to produce monoclonal antibodies, scientists have enabled a new era of targeted research and development. And as tools improve and knowledge deepens, the ability to customize and optimize cell lines will continue to unlock new biotechnological possibilities.