Loading...
In modern molecular biology, understanding cell-to-cell variability has become essential. Traditional RNA sequencing methods—though powerful—average signals from millions of cells, potentially masking rare cell types and unique transcriptional events. Single-Cell RNA Sequencing (scRNA-seq) was developed to overcome this limitation by providing gene expression profiles at single-cell resolution.
This method has reshaped research across developmental biology, immunology, neuroscience, oncology, and plant sciences. By detecting mRNA transcripts from thousands to millions of individual cells, scientists can identify new cell types, reconstruct developmental lineages, and discover dynamic regulatory mechanisms.
Gentaur
Scientific Publications
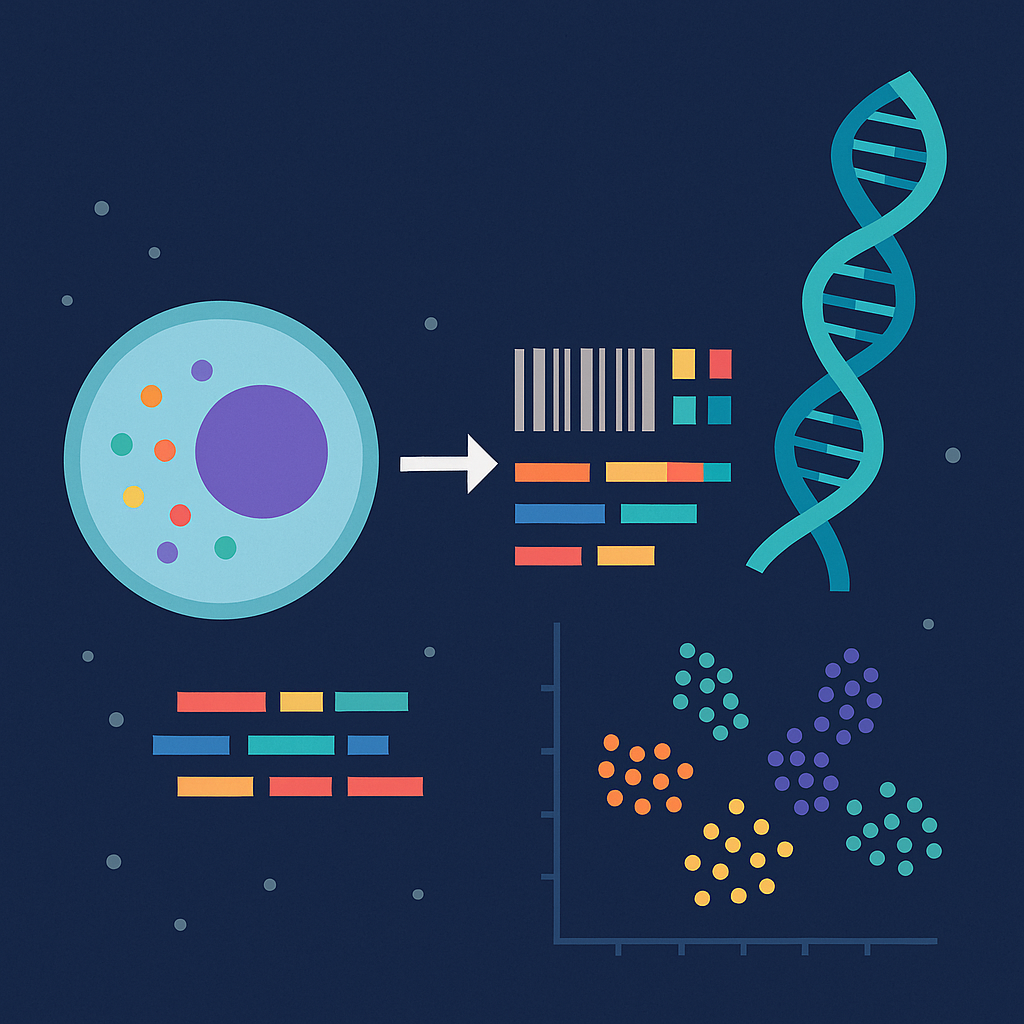
The standard scRNA-seq workflow consists of several key steps:
1. Tissue Dissociation and Single-Cell Isolation
Cells must be isolated from tissues using:
Cell isolation is achieved through:
Cell viability and integrity are crucial for downstream success.
Once individual cells are successfully isolated and placed into wells, droplets, or microfluidic chambers, the next critical step is to capture the mRNA molecules and convert them into complementary DNA (cDNA). This step forms the molecular foundation of the entire scRNA-seq workflow.
Poly-A Tailing and Oligo(dT) Priming
Most eukaryotic mRNA molecules have a polyadenylated (poly-A) tail at their 3’ ends. This tail allows selective targeting of mRNA using oligo(dT) primers, short stretches of thymidine (T) nucleotides.
These oligo(dT) primers are immobilized on beads or attached to surfaces (e.g., Gel Beads in Emulsion, or GEMs in 10x Genomics).
The primer often contains three domains:
For more Click here NIH Reference on Primer Design
Cell-Specific Barcoding
Each cell is assigned a unique barcode sequence, typically ~12–16 nucleotides long. This barcode ensures that after pooling and sequencing, the data from each cell can be distinguished during bioinformatic analysis.
This cell barcode is attached to every cDNA molecule generated from that cell’s mRNA, allowing demultiplexing of sequencing reads into distinct cells.
Unique Molecular Identifiers (UMIs)
A UMI is a short (typically 8–10 bp) random sequence that labels each individual mRNA molecule prior to PCR amplification.
Since PCR introduces bias—some molecules are overamplified—UMIs help quantify actual mRNA abundance by counting only unique sequences.
This approach eliminates duplicate artifacts, ensuring molecule-level resolution of gene expression.
Reverse Transcription (RT)
The barcoded mRNA is reverse transcribed into cDNA using reverse transcriptase enzymes. This reaction takes place:
During RT:
SMART-seq2 uses template switching during RT to capture full-length transcripts, enabling isoform analysis. This differs from 3’-tag protocols that only capture the end of transcripts.
After the reverse transcription of mRNA into barcoded cDNA, the resulting DNA molecules are still present in very low quantities—often femtograms to picograms per cell. To make them suitable for sequencing, these molecules must undergo amplification and adapter ligation to form a sequencing-compatible library.
cDNA Amplification: Turning Low-Input into High-Yield
The amplification step ensures there is enough cDNA for sequencing while maintaining quantitative accuracy. There are two main strategies depending on the scRNA-seq platform:
A. PCR-Based Amplification (UMI-Compatible)
Used in 3’-tagged methods like 10x Genomics and Drop-seq
Since each molecule carries a Unique Molecular Identifier (UMI), PCR bias does not affect quantification accuracy
Enzymes used: high-fidelity DNA polymerases such as KAPA HiFi or Q5
UMI-Aware PCR Amplification – NCBI
B. Template Switching and Full-Length cDNA Amplification
Used in Smart-seq2, which captures full-length transcripts
Involves template switching oligonucleotides (TSOs) to allow the polymerase to extend beyond the mRNA cap
Yields more complete transcript models, including isoforms
Library Preparation: Adapter Ligation and Indexing
To prepare cDNA for sequencing, platform-specific sequencing adapters must be added to the amplified DNA. These include:
Each library is quality-controlled using:
Final Output: Sequencing-Ready Library
The result is a sequencing-ready library, containing:
This library is compatible with:
Once the libraries are constructed, they are loaded onto a next-generation sequencing (NGS) platform for high-throughput sequencing. This is where analog molecular information is transformed into digital data.
Typical scRNA-seq sequencing layout includes:
This allows:
Single-cell RNA sequencing (scRNA-seq) represents a transformative pathway in scientific research by enabling the study of gene expression at the resolution of individual cells, rather than in bulk populations. This innovative method allows researchers to capture the full complexity of biological systems by revealing cellular heterogeneity, transient cell states, and rare subpopulations that were previously undetectable. By isolating single cells, barcoding their transcripts, and sequencing their mRNA profiles, scRNA-seq provides a detailed molecular fingerprint of each cell. This breakthrough has redefined how scientists explore developmental processes, immune responses, tissue regeneration, and disease mechanisms—offering a precise, unbiased, and scalable strategy to dissect the molecular architecture of complex biological systems at unprecedented depth.
Mouse embryonic scRNA-seq atlas
Single-cell RNA sequencing (scRNA-seq) is one of the most powerful tools available to modern life scientists. By offering a high-resolution lens into gene expression within individual cells, it has unlocked the ability to explore tissue complexity, developmental processes, immune responses, and more.
The field is moving quickly—integrating spatial biology, multi-omics, and AI-enhanced analysis—and as costs decline, this technology will become standard in many types of biological research.
Despite its strengths, scRNA-seq faces several limitations:
Batch correction tools like Harmony and Combat are commonly used to reduce artifacts.