Red Fluorescent SR In vivo Poly (active) Caspase (VAD) Assay
CAT:
436-983
Size:
24 Tests
Price:
Ask
- Availability: 24/48H Stock Items & 2 to 6 Weeks non Stock Items.
- Dry Ice Shipment: No







Red Fluorescent SR In vivo Poly (active) Caspase (VAD) Assay
Description:
A non-cytotoxic, cell-permeant fluorescent inhibitor of caspases optimized for use in whole live animals. The probe contains the preferred binding sequence for most caspases (Val-Ala-Asp or VAD), coupled to a SR dye and a FMK reactive entity.Specifications:
SR-FLIVO® Poly Caspase Inhibitor (SR-VAD-FMK)Target:
Poly CaspasesLabel:
ICTCell Type:
COLO205Type:
Cell ViabiityDetection Method:
Fluorescence Microscope, Flow Cytometer, Window Chamber SystemWavelength:
550-580 nm/590-600 nmAssay Protocol:
1. Prepare samples and controls, 2. Dilute cellular wash buffer 1:10 with diH2O, 3. Reconstitute FLISP with 50 µL DMSO, 4. Dilute FLISP 1:5 by adding 200 µL PBS, 5. Add 10 µL FLISP to each sample (~500 µL aliquot of cultured cells), 6. Incubate ~ 1 hour., 7. Remove media and wash cells: add wash buffer and spin cells (twice); or add fresh media and incubate 1 hour, 8. If desired, label with additional stains, such as Hoechst, PI, or an antibody, 9. If desired, fix or embed cells, 10. Analyze with a fluorescence microscope, fluorescence plate reader, or flow cytometer. FAM-FLISP excites at 488 nm and emits at 520 nm.Sample Type:
Whole live animal, excised tissueComponents:
Kit 983 24 Tests:, • SR-FLIVO® Poly Caspase Inhibitor (SR-VAD-FMK), 4 vials, #6219, • 10X Injection Buffer, 5 mL, #6220, • Kit ManualShipping Conditions:
Ships overnight (domestic), International Priority ShippingStorage Temperature:
2-8°CCellular Imaging & Detection:
FLIVOTarget Description:
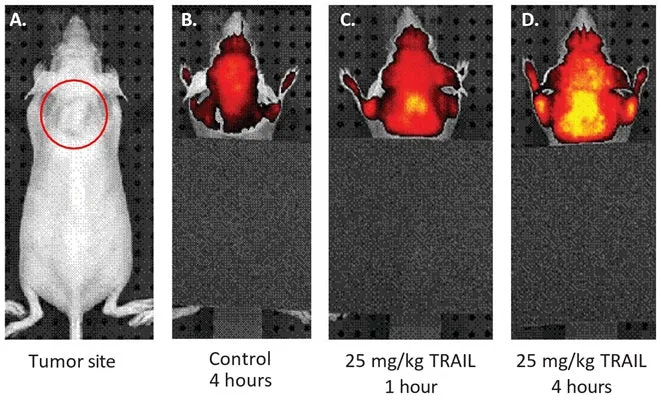
Similar to our FLICA® probes, but optimized for whole live animal imaging, SR in vivo probes are non-cytotoxic fluorescent inhibitors of caspases. ICT’s Red Fluorescent SR In vivo Poly (active) Caspase (VAD) Assay inhibitor probe contains the preferred binding sequence for all caspases, Val-Ala-Asp (VAD). This preferred poly caspase tripeptide binding sequence is labeled at the amino terminus end with a sulforhodamine B (SR) dye and linked at the carboxyl end to a fluoromethyl ketone (FMK) reactive entity. The resulting cell permeant, fluorescent molecule, SR-VAD-FMK, optimally excites at 550-580 nm and emits at 590-600 nm. The spectra for the probe is shown in Figure 1. Apoptosis is an evolutionarily conserved process of programmed cell suicide. It is centered on a cascade of proteolytic enzymes called caspases that are triggered in response to pro-apoptotic signals. Like most other proteases, caspases are synthesized as pro-form precursors that undergo proteolytic maturation, either autocatalytically or in a cascade by enzymes with similar specificity. Active caspase enzymes consist of two large (~20 kD) and two small (~10 kD) subunits that non-covalently associate to form a two heterodimer, tetrameric active caspase. Once activated, caspases cleave protein substrates leading to the eventual disassembly of the cell. Caspases have been identified in organisms ranging from C. elegans to humans. Mammalian caspases play distinct roles in both apoptosis and inflammation. The kit provides a simple yet accurate method to detect caspase activity in vivo. To label cells containing elevated levels of active caspases, inject the probe intravenously and let it circulate ~60 minutes. Because the reagent is cell-permeant, it readily diffuses in and out of all cells it encounters as it circulates throughout the body. If there are active caspase enzymes inside a cell, the reagent will form an irreversible covalent bond with a reactive cysteine on the large subunit of the caspase heterodimer, thereby inhibiting further enzymatic activity. The bound reagent probe will remain inside the cell as long as the cell membrane is intact. Any unbound probe is removed from the circulation of the animal in about an hour. Additional time may be needed for the probe to clear other tissues. The remaining red fluorescent signal in the tissue after unbound probe has cleared is a direct measure of caspase activity that occurred at the time the reagent was injected. Apoptotic cells will retain a higher concentration of the probe and fluoresce brighter than non-apoptotic cells. There is no interference from pro-caspases or inactive forms of the enzyme. If the treatment is causing cell death via apoptosis, apoptotic cells will have an elevated level of caspase activity relative to non-apoptotic or negative control cells and fluoresce red with the probe. After labeling with probe, excised tissues can be counter-stained with other reagents and fixed or frozen. Once the animals have been injected with probe and excess unbound probe has cleared from the body of the animal, the tissues are ready for analysis and no further staining is necessary. Because the probe is a direct stain, it eliminates any false positives that may arise from manipulation of the tissue. This gives a true representation of the induction of apoptosis in vivo as a result of the experimental condition. Tissues can be viewed directly through a window chamber system or other accessible cavity, or thin tissue sections can be prepared after sacrificing the animal. Tissues labeled with probe can be counter-stained with other reagents such as DAPI and fixed or frozen for future analysis. The fluorescence intensity can be quantified by excising the tissue and analyzing cells with a flow cytometer. The probe optimally excites at 550-580 nm and has a peak emission at 590-600 nm (Figure 1).